Analysis of eDNA involves the collection of samples from all kinds of environment such as water, soil, sediment, feces, etc and from where nucleic acid, DNA or RNA is extracted. These extracted nucleotides can be the basis for a range of eDNA analyses:
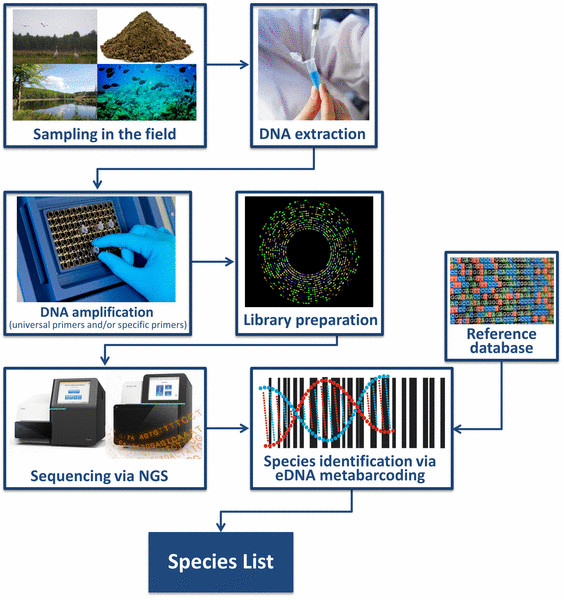
The department houses facilities for high throughput DNA/RNA extraction even using robots, QPCR, library preparation and next generation sequencing facilities. We have our sequencing facilities including Nanopore, NextSeq, MiSeq platform to support participation in all types of projects involving analysis of eDNA. This includes identification of single organisms and entire communities in various matrices, their expression of genes relevant for provision of important ecosystem services and health of humans, animals and plants.
Computers handle analysis of big-data from the DNA sequencing platform with large computation capacity. The center consist of in house servers for bioinformatics and data analysis of big data.

QPCR machines

Nanopore and Illumina MiSeq- and NextSeq-sequencing platforms for analysing eDNA